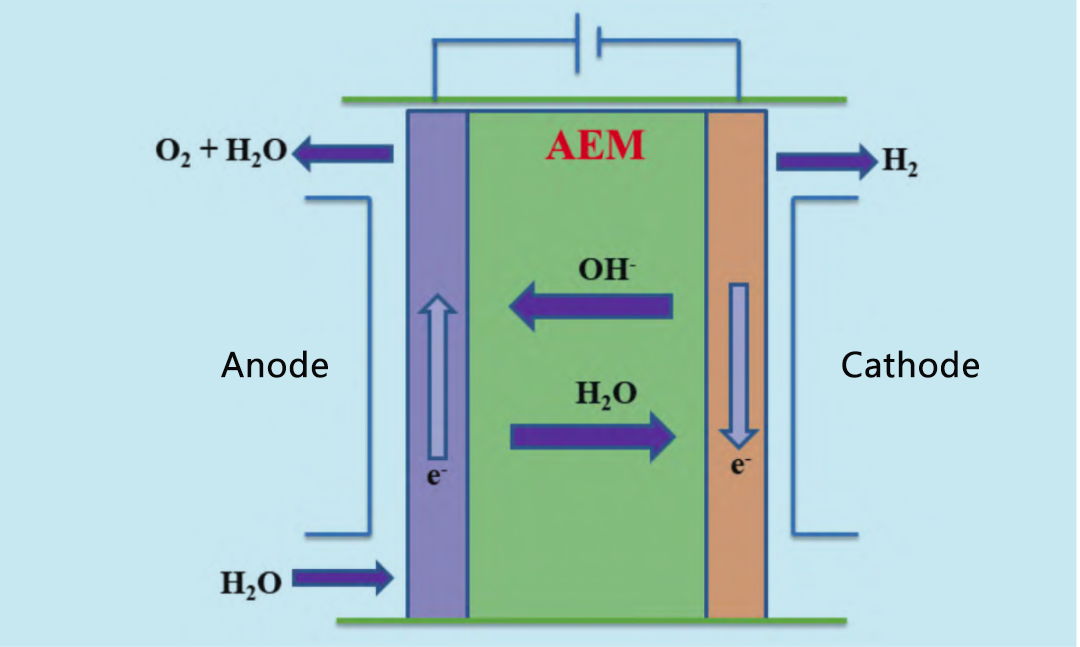
ʻO AEM ma kekahi ʻano he hybrid o PEM a me ka diaphragm maʻamau e pili ana i ka lye electrolysis. Hōʻike ʻia ke kumumanaʻo o AEM electrolytic cell ma ka Figure 3. Ma ka cathode, hoʻemi ʻia ka wai e hana i ka hydrogen a me OH -. OH - kahe i loko o ka diaphragm i ka anode, kahi e hui hou ai e hana i ka oxygen.
Li et al. [1-2] ua aʻo i ka polystyrene quaternized a me ka polyphenylene AEM kiʻekiʻe-performance wai electrolyzer, a ua hōʻike nā hualoaʻa he 2.7A/cm2 ka mānoanoa o kēia manawa ma 85°C ma ka volta o 1.8V. I ka hoʻohana ʻana iā NiFe a me PtRu/C ma ke ʻano he catalysts no ka hana hydrogen, ua emi nui ka nui o kēia manawa i 906mA/cm2. ʻO Chen et al. [5] ua aʻo i ka hoʻohana ʻana i ka mea hoʻoheheʻe electrolytic kiʻekiʻe ʻole noble metala ma ka alkaline polymer film electrolyzer. Ua ho'ēmiʻia nā'okikene NiMo e nā kinoea H2/NH3, NH3, H2 a me N2 i nā mahana likeʻole e synthesize i nā mea hoʻoheheʻe hydrogen electrolytic. Hōʻike nā hopena i ka hana maikaʻi loa o ka NiMo-NH3/H2 catalyst me H2/NH3 hōʻemi, me ka nui o kēia manawa a hiki i 1.0A/cm2 a me ka hoʻololi ʻana i ka ikehu o 75% ma 1.57V a me 80°C. ʻO Evonik Industries, ma muli o kāna ʻenehana membrane hoʻokaʻawale kinoea, ua hoʻomohala i kahi mea polymer patented no ka hoʻohana ʻana i nā cell electrolytic AEM a ke hoʻonui nei i ka hana membrane ma kahi laina pailaka. ʻO ka hana aʻe e hōʻoia i ka hilinaʻi o ka ʻōnaehana a hoʻomaikaʻi i nā kikoʻī pākaukau, ʻoiai e hoʻonui ana i ka hana.
I kēia manawa, ʻo nā pilikia nui e kū nei i nā cell electrolytic AEM ʻo ka nele o ka conductivity kiʻekiʻe a me ke kūpaʻa alkaline o AEM, a ʻo ka electrocatalyst metala makamae e hoʻonui i ke kumukūʻai o nā mea hana electrolytic. I ka manawa like, e hoʻemi ana ka CO2 i ke kiʻi kiʻiʻoniʻoni i ka pale kiʻiʻoniʻoni a me ke kūpaʻa electrode, no laila e hōʻemi i ka hana electrolytic. ʻO ke kuhikuhi o ka hoʻomohala ʻana o AEM electrolyzer e like me kēia: 1. Hoʻomohala i ka AEM me ke kiʻekiʻe conductivity, koho ion a me ka paʻa alkaline lōʻihi. 2. E lanakila i ka pilikia o ke kumukūʻai kiʻekiʻe o ka catalyst metala makamae, e hoʻomohala i ka catalyst me ka ʻole o ka metala makamae a me ka hana kiʻekiʻe. 3. I kēia manawa, ʻo ke kumu kūʻai o ka AEM electrolyzer he $ 20 / m2, pono e hoʻemi ʻia ma o nā kumu waiwai liʻiliʻi a me nā ʻanuʻu synthesis hoʻemi, i mea e hōʻemi ai i ke kumukūʻai holoʻokoʻa o AEM electrolyzer. 4. E ho'ēmi i ka CO2 maʻiʻo i loko o ka electrolytic cell a hoʻomaikaʻi i ka hana electrolytic.
[1] Liu L, Kohl P A. Anion e alakaʻi ana i nā copolymers multiblock me nā cations hoʻopili like ʻole [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2018, 56(13): 1395 — 1403.
[2] Li D, Park EJ, Zhu W, a me al. ʻO nā ionomers polystyrene quaternized kiʻekiʻe no ka hana kiʻekiʻe o ka hoʻololi anion hoʻololi i ka wai electrolysers [J]. Nature Energy, 2020, 5: 378 — 385.
Ka manawa hoʻouna: Feb-02-2023