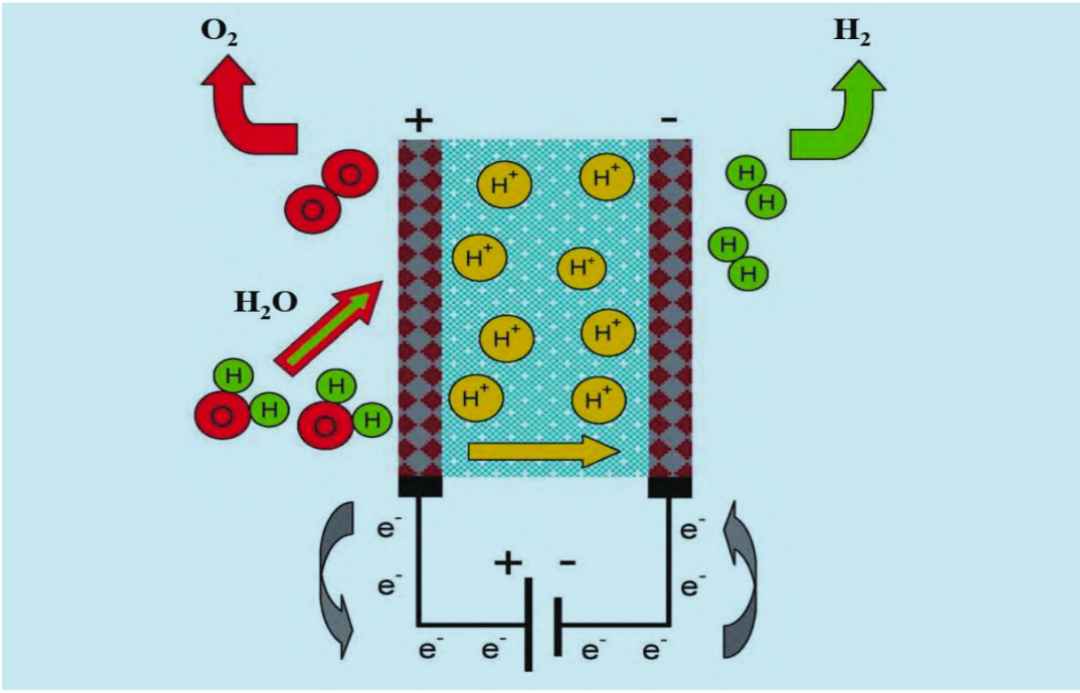
In 1966, General Electric Company developed water electrolytic cell based on proton conduction concept, using polymer membrane as electrolyte. PEM cells were commercialized by General Electric in 1978. Currently, the company produces fewer PEM cells, mainly because of its limited hydrogen production, short life and high investment cost. A PEM cell has a bipolar structure, and electrical connections between cells are made through bipolar plates, which play an important role in discharging the generated gases. The anode, cathode, and membrane group form the membrane electrode assembly (MEA). The electrode is usually composed of precious metals such as platinum or iridium. At the anode, water is oxidized to produce oxygen, electrons and protons. At the cathode, the oxygen, electrons and protons produced by the anode circulate through the membrane to the cathode, where they are reduced to produce hydrogen gas. The principle of PEM electrolyzer is shown in the figure.
PEM electrolytic cells are usually used for small-scale hydrogen production, with a maximum hydrogen production of about 30Nm3/h and a power consumption of 174kW. Compared with alkaline cell, the actual hydrogen production rate of PEM cell almost covers the whole limit range. The PEM cell can work at A higher current density than the alkaline cell, even up to 1.6A/cm2, and the electrolytic efficiency is 48%-65%. Because the polymer film is not resistant to high temperature, the temperature of the electrolytic cell is often below 80°C. Hoeller electrolyzer has developed an optimized cell surface technology for small PEM electrolyzers. The cells can be designed according to the requirements, reducing the amount of precious metals and increasing the operating pressure. The main advantage of PEM electrolyzer is that the hydrogen production changes almost synchronously with the energy supplied, which is suitable for the change of hydrogen demand. Hoeller cells respond to 0-100% load rating changes in seconds. Hoeller’s patented technology is undergoing validation tests, and the test facility will be built by the end of 2020.
The purity of hydrogen produced by PEM cells can be as high as 99.99%, which is higher than that of alkaline cells. In addition, the extremely low gas permeability of the polymer membrane reduces the risk of forming flammable mixtures, allowing the electrolyzer to operate at extremely low current densities. The conductivity of the water supplied to the electrolyzer must be less than 1S/cm. Because proton transport across the polymer membrane responds quickly to power fluctuations, PEM cells can operate in different power supply modes. Although the PEM cell has been commercialized, it has some disadvantages, mainly the high investment cost and the high expense of both membrane and precious metal based electrodes. In addition, the service life of PEM cells is shorter than that of alkaline cells. In the future, the capacity of PEM cell to produce hydrogen needs to be greatly improved.
Post time: Feb-02-2023