Welcome to our website for product information and consultation.
Our website: https://www.vet-china.com/
Physical and chemical activation method
Physical and chemical activation method refers to the method of preparing porous materials by combining the above two activation methods. Generally, chemical activation is performed first, and then physical activation is performed. Firstly soak cellulose in 68%~85% H3PO4 solution at 85℃ for 2h, then carbonized it in a muffle furnace for 4h, and then activated it with CO2. The specific surface area of the activated carbon obtained was as high as 3700m2·g-1. Try to use sisal fiber as raw material, and activated the activated carbon fiber (ACF) obtained by H3PO4 activation once, heated it to 830℃ under N2 protection, and then used water vapor as an activator for secondary activation. The specific surface area of ACF obtained after 60min of activation was significantly improved.
Characterization of pore structure performance of activated carbon
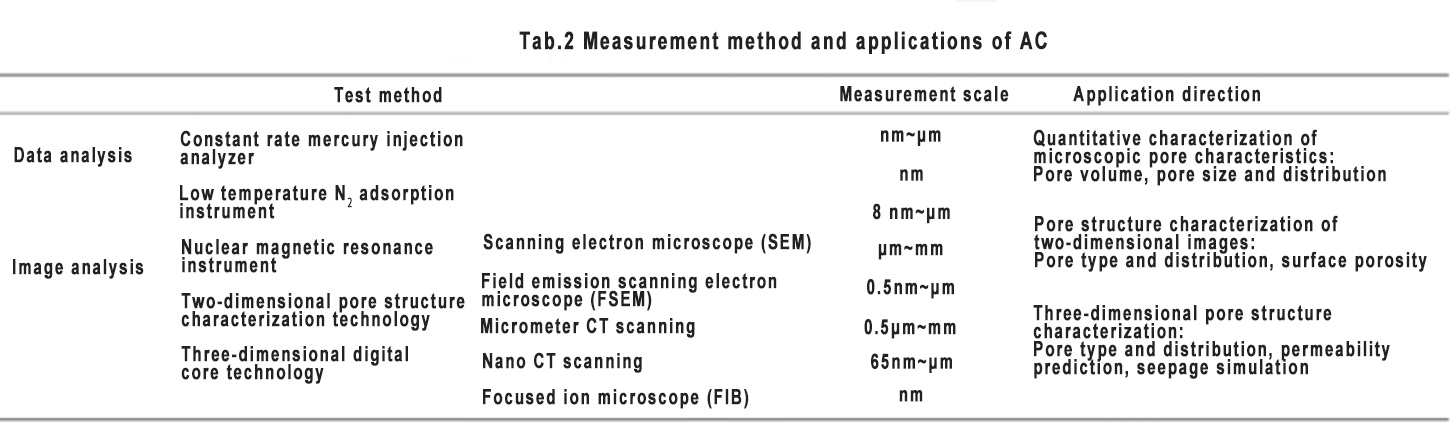
Commonly used activated carbon performance characterization methods and application directions are shown in Table 2. The pore structure characteristics of the material can be tested from two aspects: data analysis and image analysis.
Research progress of pore structure optimization technology of activated carbon
Although activated carbon has rich pores and huge specific surface area, it has excellent performance in many fields. However, due to its wide raw material selectivity and complex preparation conditions, the finished products generally have the disadvantages of chaotic pore structure, different specific surface area, disordered pore size distribution, and limited surface chemical properties. Therefore, there are disadvantages such as large dosage and narrow adaptability in the application process, which cannot meet the market requirements. Therefore, it is of great practical significance to optimize and regulate the structure and improve its comprehensive utilization performance. Commonly used methods for optimizing and regulating pore structure include chemical regulation, polymer blending, and catalytic activation regulation.
Chemical regulation technology
Chemical regulation technology refers to the process of secondary activation (modification) of porous materials obtained after activation with chemical reagents, eroding the original pores, expanding the micropores, or further creating new micropores to increase the specific surface area and pore structure of the material. Generally speaking, the finished product of one activation is generally immersed in 0.5~4 times of chemical solution to regulate the pore structure and increase the specific surface area. All kinds of acid and alkali solutions can be used as reagents for secondary activation.
Acid surface oxidation modification technology
Acid surface oxidation modification is a commonly used regulation method. At an appropriate temperature, acid oxidants can enrich the pores inside activated carbon, improve its pore size, and dredge blocked pores. At present, domestic and foreign research mainly focuses on the modification of inorganic acids. HN03 is a commonly used oxidant, and many scholars use HN03 to modify activated carbon. Tong Li et al. [28] found that HN03 can increase the content of oxygen-containing and nitrogen-containing functional groups on the surface of activated carbon and improve the adsorption effect of mercury.
Modifying activated carbon with HN03, after modification, the specific surface area of activated carbon decreased from 652m2·g-1 to 241m2·g-1, the average pore size increased from 1.27nm to 1.641nm, and the adsorption capacity of benzophenone in simulated gasoline increased by 33.7%. Modifying wood activated carbon with 10% and 70% volume concentration of HN03, respectively. The results show that the specific surface area of activated carbon modified with 10% HN03 increased from 925.45m2·g-1 to 960.52m2·g-1; after modification with 70% HN03, the specific surface area decreased to 935.89m2·g-1. The removal rates of Cu2+ by activated carbon modified with two concentrations of HN03 were above 70% and 90%, respectively.
For activated carbon used in the adsorption field, the adsorption effect depends not only on the pore structure but also on the surface chemical properties of the adsorbent. The pore structure determines the specific surface area and adsorption capacity of activated carbon, while the surface chemical properties affect the interaction between activated carbon and adsorbate. Finally it was found that acid modification of activated carbon can not only adjust the pore structure inside the activated carbon and clear the blocked pores, but also increase the content of acidic groups on the surface of the material and enhance the polarity and hydrophilicity of the surface. The adsorption capacity of EDTA by activated carbon modified by HCI increased by 49.5% compared with that before modification, which was better than that of HNO3 modification.
Modified commercial activated carbon with HNO3 and H2O2 respectively! The specific surface areas after modification were 91.3% and 80.8% of those before modification, respectively. New oxygen-containing functional groups such as carboxyl, carbonyl and phenol were added to the surface. The adsorption capacity of nitrobenzene by HNO3 modification was the best, which was 3.3 times that before modification.It is found that the increase in the content of oxygen-containing functional groups in activated carbon after acid modification led to an increase in the number of surface active points, which had a direct effect on improving the adsorption capacity of the target adsorbate.
Compared with inorganic acids, there are few reports on the organic acid modification of activated carbon. Compare the effects of organic acid modification on the pore structure properties of activated carbon and the adsorption of methanol. After modification, the specific surface area and total pore volume of activated carbon decreased. The stronger the acidity, the greater the decrease. After modification with oxalic acid, tartaric acid and citric acid, the specific surface area of activated carbon decreased from 898.59m2·g-1 to 788.03m2·g-1, 685.16m2·g-1 and 622.98m2·g-1 respectively. However, the microporosity of activated carbon increased after modification. The microporosity of activated carbon modified with citric acid increased from 75.9% to 81.5%.
Oxalic acid and tartaric acid modification are beneficial to the adsorption of methanol, while citric acid has an inhibitory effect. However, J.Paul Chen et al. [35] found that activated carbon modified with citric acid can enhance the adsorption of copper ions. Lin Tang et al. [36] modified commercial activated carbon with formic acid, oxalic acid and aminosulfonic acid. After modification, the specific surface area and pore volume were reduced. Oxygen-containing functional groups such as 0-HC-0, C-0 and S=0 were formed on the surface of the finished product, and uneven etched channels and white crystals appeared. The equilibrium adsorption capacity of acetone and isopropanol also increased significantly.
Alkaline solution modification technology
Some scholars also used alkaline solution to perform secondary activation on activated carbon. Impregnate homemade coal-based activated carbon with Na0H solution of different concentrations to control the pore structure. The results showed that a lower alkali concentration was conducive to pore increase and expansion. The best effect was achieved when the mass concentration was 20%. The activated carbon had the highest specific surface area (681m2·g-1) and pore volume (0.5916cm3·g-1). When the mass concentration of Na0H exceeds 20%, the pore structure of activated carbon is destroyed and the pore structure parameters begin to decrease. This is because the high concentration of Na0H solution will corrode the carbon skeleton and a large number of pores will collapse.
Preparing high-performance activated carbon by polymer blending. The precursors were furfural resin and furfuryl alcohol, and ethylene glycol was the pore-forming agent. The pore structure was controlled by adjusting the content of the three polymers, and a porous material with a pore size between 0.008 and 5 μm was obtained. Some scholars have proved that polyurethane-imide film (PUI) can be carbonized to obtain carbon film, and the pore structure can be controlled by changing the molecular structure of polyurethane (PU) prepolymer [41]. When PUI is heated to 200°C, PU and polyimide (PI) will be generated. When the heat treatment temperature rises to 400°C, PU pyrolysis produces gas, resulting in the formation of a pore structure on the PI film. After carbonization, a carbon film is obtained. In addition, the polymer blending method can also improve some physical and mechanical properties of the material to a certain extent
Catalytic activation regulation technology
Catalytic activation regulation technology is actually a combination of chemical activation method and high-temperature gas activation method. Generally, chemical substances are added to the raw materials as catalysts, and the catalysts are used to assist the carbonization or activation process to obtain porous carbon materials. Generally speaking, metals generally have catalytic effects, but the catalytic effects vary.
In fact, there is usually no obvious boundary between chemical activation regulation and catalytic activation regulation of porous materials. This is because both methods add reagents during the carbonization and activation process. The specific role of these reagents determines whether the method belongs to the category of catalytic activation.
The structure of the porous carbon material itself, the physical and chemical properties of the catalyst, the catalytic reaction conditions and the catalyst loading method can all have different degrees of influence on the regulation effect. Using bituminous coal as raw material, Mn(N03)2 and Cu(N03)2 as catalysts can prepare porous materials containing metal oxides. The appropriate amount of metal oxides can improve the porosity and pore volume, but the catalytic effects of different metals are slightly different. Cu(N03)2 can promote the development of pores in the range of 1.5~2.0nm. In addition, the metal oxides and inorganic salts contained in the raw material ash will also play a catalytic role in the activation process. Xie Qiang et al. [42] believed that the catalytic activation reaction of elements such as calcium and iron in inorganic matter can promote the development of pores. When the content of these two elements is too high, the proportion of medium and large pores in the product increases significantly.
Conclusion
Although activated carbon, as the most widely used green porous carbon material, has played an important role in industry and life, it still has great potential for improvement in raw material expansion, cost reduction, quality improvement, energy improvement, life extension and strength improvement. Finding high-quality and cheap activated carbon raw materials, developing clean and efficient activated carbon production technology, and optimizing and regulating the pore structure of activated carbon according to different application fields will be an important direction for improving the quality of activated carbon products and promoting the high-quality development of the activated carbon industry.
Post time: Aug-27-2024