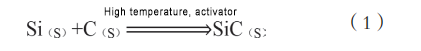In the silicon carbide single crystal growth process, physical vapor transport is the current mainstream industrialization method. For the PVT growth method, silicon carbide powder has a great influence on the growth process. All parameters of silicon carbide powder directly affect the quality of single crystal growth and electrical properties. In current industrial applications, the commonly used silicon carbide powder synthesis process is the self-propagating high-temperature synthesis method.
The self-propagating high-temperature synthesis method uses high temperature to give the reactants initial heat to start chemical reactions, and then uses its own chemical reaction heat to allow the unreacted substances to continue to complete the chemical reaction. However, since the chemical reaction of Si and C releases less heat, other reactants must be added to maintain the reaction. Therefore, many scholars have proposed an improved self-propagating synthesis method on this basis, introducing an activator. The self-propagating method is relatively easy to implement, and various synthesis parameters are easy to stably control. Large-scale synthesis meets the needs of industrialization.
As early as 1999, Bridgeport used the self-propagating high-temperature synthesis method to synthesize SiC powder, but it used ethoxysilane and phenol resin as raw materials, which was costly. Gao Pan and others used high-purity Si powder and C powder as raw materials to synthesize SiC powder by high-temperature reaction in an argon atmosphere. Ning Lina prepared large-particle SiC powder by secondary synthesis.
The medium-frequency induction heating furnace developed by the Second Research Institute of China Electronics Technology Group Corporation evenly mixes silicon powder and carbon powder in a certain stoichiometric ratio and places them in a graphite crucible. The graphite crucible is placed in a medium-frequency induction heating furnace for heating, and the temperature change is used to synthesize and transform the low-temperature phase and high-temperature phase silicon carbide respectively. Since the temperature of the β-SiC synthesis reaction in the low-temperature phase is lower than the volatilization temperature of Si, the synthesis of β-SiC under high vacuum can well ensure the self-propagation. The method of introducing argon, hydrogen and HCl gas in the synthesis of α-SiC prevents the decomposition of SiC powder in the high-temperature stage, and can effectively reduce the nitrogen content in α-SiC powder.
Shandong Tianyue designed a synthesis furnace, using silane gas as silicon raw material and carbon powder as carbon raw material. The amount of raw material gas introduced was adjusted by a two-step synthesis method, and the final synthesized silicon carbide particle size was between 50 and 5 000 um.
1 Control factors of powder synthesis process
1.1 Effect of powder particle size on crystal growth
The particle size of silicon carbide powder has a very important influence on the subsequent single crystal growth. The growth of SiC single crystal by PVT method is mainly achieved by changing the molar ratio of silicon and carbon in the gas phase component, and the molar ratio of silicon and carbon in the gas phase component is related to the particle size of silicon carbide powder. The total pressure and silicon-carbon ratio of the growth system increase with the decrease of particle size. When the particle size decreases from 2-3 mm to 0.06 mm, the silicon-carbon ratio increases from 1.3 to 4.0. When the particles are small to a certain extent, the Si partial pressure increases, and a layer of Si film is formed on the surface of the growing crystal, inducing gas-liquid-solid growth, which affects the polymorphism, point defects and line defects in the crystal. Therefore, the particle size of high-purity silicon carbide powder must be well controlled.
In addition, when the size of SiC powder particles is relatively small, the powder decomposes faster, resulting in excessive growth of SiC single crystals. On the one hand, in the high-temperature environment of SiC single crystal growth, the two processes of synthesis and decomposition are carried out simultaneously. Silicon carbide powder will decompose and form carbon in the gas phase and solid phase such as Si, Si2C, SiC2, resulting in serious carbonization of polycrystalline powder and the formation of carbon inclusions in the crystal; on the other hand, when the decomposition rate of the powder is relatively fast, the crystal structure of the grown SiC single crystal is prone to change, making it difficult to control the quality of the grown SiC single crystal.
1.2 Effect of powder crystal form on crystal growth
The growth of SiC single crystal by PVT method is a sublimation-recrystallization process at high temperature. The crystal form of SiC raw material has an important influence on crystal growth. In the process of powder synthesis, the low-temperature synthesis phase (β-SiC) with a cubic structure of the unit cell and the high-temperature synthesis phase (α-SiC) with a hexagonal structure of the unit cell will be mainly produced. There are many silicon carbide crystal forms and a narrow temperature control range. For example, 3C-SiC will transform into hexagonal silicon carbide polymorph, i.e. 4H/6H-SiC, at temperatures above 1900°C.
During the single crystal growth process, when β-SiC powder is used to grow crystals, the silicon-carbon molar ratio is greater than 5.5, while when α-SiC powder is used to grow crystals, the silicon-carbon molar ratio is 1.2. When the temperature rises, a phase transition occurs in the crucible. At this time, the molar ratio in the gas phase becomes larger, which is not conducive to crystal growth. In addition, other gas phase impurities, including carbon, silicon, and silicon dioxide, are easily generated during the phase transition process. The presence of these impurities causes the crystal to breed microtubes and voids. Therefore, the powder crystal form must be precisely controlled.
1.3 Effect of powder impurities on crystal growth
The impurity content in SiC powder affects the spontaneous nucleation during crystal growth. The higher the impurity content, the less likely it is for the crystal to spontaneously nucleate. For SiC, the main metal impurities include B, Al, V, and Ni, which may be introduced by processing tools during the processing of silicon powder and carbon powder. Among them, B and Al are the main shallow energy level acceptor impurities in SiC, resulting in a decrease in SiC resistivity. Other metal impurities will introduce many energy levels, resulting in unstable electrical properties of SiC single crystals at high temperatures, and have a greater impact on the electrical properties of high-purity semi-insulating single crystal substrates, especially the resistivity. Therefore, high-purity silicon carbide powder must be synthesized as much as possible.
1.4 Effect of nitrogen content in powder on crystal growth
The level of nitrogen content determines the resistivity of the single crystal substrate. Major manufacturers need to adjust the nitrogen doping concentration in the synthetic material according to the mature crystal growth process during powder synthesis. High-purity semi-insulating silicon carbide single crystal substrates are the most promising materials for military core electronic components. To grow high-purity semi-insulating single crystal substrates with high resistivity and excellent electrical properties, the content of the main impurity nitrogen in the substrate must be controlled at a low level. Conductive single crystal substrates require nitrogen content to be controlled at a relatively high concentration.
2 Key control technology for powder synthesis
Due to the different use environments of silicon carbide substrates, the synthesis technology for growth powders also has different processes. For N-type conductive single crystal growth powders, high impurity purity and single phase are required; while for semi-insulating single crystal growth powders, strict control of nitrogen content is required.
2.1 Powder particle size control
2.1.1 Synthesis temperature
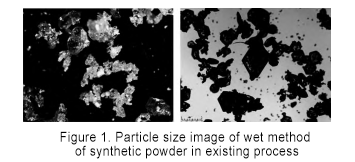
Keeping other process conditions unchanged, SiC powders generated at synthesis temperatures of 1900 ℃, 2000 ℃, 2100 ℃, and 2200 ℃ were sampled and analyzed. As shown in Figure 1, it can be seen that the particle size is 250~600 μm at 1900 ℃, and the particle size increases to 600~850 μm at 2000 ℃, and the particle size changes significantly. When the temperature continues to rise to 2100 ℃, the particle size of SiC powder is 850~2360 μm, and the increase tends to be gentle. The particle size of SiC at 2200 ℃ is stable at about 2360 μm. The increase in synthesis temperature from 1900 ℃ has a positive effect on the SiC particle size. When the synthesis temperature continues to increase from 2100 ℃, the particle size no longer changes significantly. Therefore, when the synthesis temperature is set to 2100 ℃, a larger particle size can be synthesized at a lower energy consumption.
2.1.2 Synthesis time
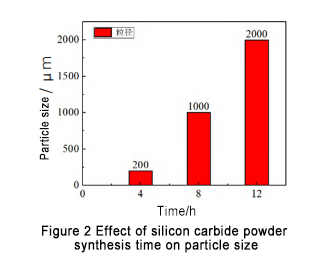
Other process conditions remain unchanged, and the synthesis time is set to 4 h, 8 h, and 12 h respectively. The generated SiC powder sampling analysis is shown in Figure 2. It is found that the synthesis time has a significant effect on the particle size of SiC. When the synthesis time is 4 h, the particle size is mainly distributed at 200 μm; when the synthesis time is 8 h, the synthetic particle size increases significantly, mainly distributed at about 1 000 μm; as the synthesis time continues to increase, the particle size increases further, mainly distributed at about 2 000 μm.
2.1.3 Influence of raw material particle size
As the domestic silicon material production chain is gradually improved, the purity of silicon materials is also further improved. At present, the silicon materials used in synthesis are mainly divided into granular silicon and powdered silicon, as shown in Figure 3.
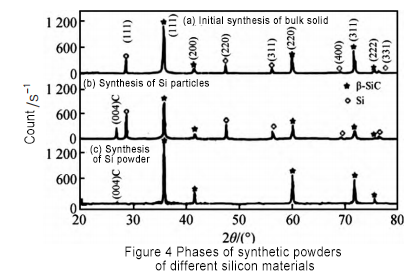
Different silicon raw materials were used to conduct silicon carbide synthesis experiments. The comparison of the synthetic products is shown in Figure 4. Analysis shows that when using block silicon raw materials, a large amount of Si elements are present in the product. After the silicon block is crushed for the second time, the Si element in the synthetic product is significantly reduced, but it still exists. Finally, silicon powder is used for synthesis, and only SiC is present in the product. This is because in the production process, large-size granular silicon needs to undergo surface synthesis reaction first, and silicon carbide is synthesized on the surface, which prevents the internal Si powder from further combining with C powder. Therefore, if block silicon is used as raw material, it needs to be crushed and then subjected to secondary synthesis process to obtain silicon carbide powder for crystal growth.
2.2 Powder crystal form control
2.2.1 Influence of synthesis temperature
Maintaining other process conditions unchanged, the synthesis temperature is 1500℃, 1700℃, 1900℃, and 2100℃, and the generated SiC powder is sampled and analyzed. As shown in Figure 5, β-SiC is earthy yellow, and α-SiC is lighter in color. By observing the color and morphology of the synthesized powder, it can be determined that the synthesized product is β-SiC at temperatures of 1500℃ and 1700℃. At 1900℃, the color becomes lighter, and hexagonal particles appear, indicating that after the temperature rises to 1900℃, a phase transition occurs, and part of β-SiC is converted into α-SiC; when the temperature continues to rise to 2100℃, it is found that the synthesized particles are transparent, and α-SiC has basically been converted.
2.2.2 Effect of synthesis time
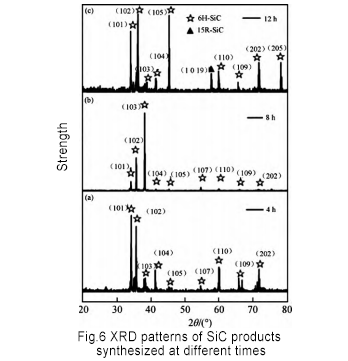
Other process conditions remain unchanged, and the synthesis time is set to 4h, 8h, and 12h, respectively. The generated SiC powder is sampled and analyzed by diffractometer (XRD). The results are shown in Figure 6. The synthesis time has a certain influence on the product synthesized by SiC powder. When the synthesis time is 4 h and 8 h, the synthetic product is mainly 6H-SiC; when the synthesis time is 12 h, 15R-SiC appears in the product.
2.2.3 Influence of raw material ratio
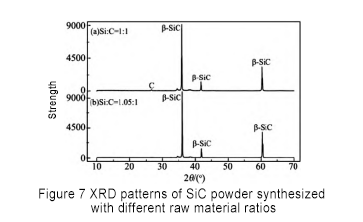
Other processes remain unchanged, the amount of silicon-carbon substances is analyzed, and the ratios are 1.00, 1.05, 1.10 and 1.15 respectively for synthesis experiments. The results are shown in Figure 7.
From the XRD spectrum, it can be seen that when the silicon-carbon ratio is greater than 1.05, excess Si appears in the product, and when the silicon-carbon ratio is less than 1.05, excess C appears. When the silicon-carbon ratio is 1.05, the free carbon in the synthetic product is basically eliminated, and no free silicon appears. Therefore, the amount ratio of silicon-carbon ratio should be 1.05 to synthesize high-purity SiC.
2.3 Control of low nitrogen content in powder
2.3.1 Synthetic raw materials
The raw materials used in this experiment are high-purity carbon powder and high-purity silicon powder with a median diameter of 20 μm. Due to their small particle size and large specific surface area, they are easy to absorb N2 in the air. When synthesizing the powder, it will be brought into the crystal form of the powder. For the growth of N-type crystals, the uneven doping of N2 in the powder leads to uneven resistance of the crystal and even changes in the crystal form. The nitrogen content of the synthesized powder after hydrogen is introduced is significantly low. This is because the volume of hydrogen molecules is small. When the N2 adsorbed in the carbon powder and silicon powder is heated and decomposed from the surface, H2 fully diffuses into the gap between the powders with its small volume, replacing the position of N2, and N2 escapes from the crucible during the vacuum process, achieving the purpose of removing the nitrogen content.
2.3.2 Synthesis process
During the synthesis of silicon carbide powder, since the radius of carbon atoms and nitrogen atoms is similar, nitrogen will replace carbon vacancies in silicon carbide, thereby increasing the nitrogen content. This experimental process adopts the method of introducing H2, and H2 reacts with carbon and silicon elements in the synthesis crucible to generate C2H2, C2H, and SiH gases. The carbon element content increases through gas phase transmission, thereby reducing carbon vacancies. The purpose of removing nitrogen is achieved.
2.3.3 Process background nitrogen content control
Graphite crucibles with large porosity can be used as additional C sources to absorb Si vapor in the gas phase components, reduce Si in the gas phase components, and thus increase C/Si. At the same time, graphite crucibles can also react with Si atmosphere to generate Si2C, SiC2 and SiC, which is equivalent to Si atmosphere bringing C source from graphite crucible into the growth atmosphere, increasing the C ratio, and also increasing carbon-silicon ratio. Therefore, the carbon-silicon ratio can be increased by using graphite crucibles with large porosity, reducing carbon vacancies, and achieving the purpose of removing nitrogen.
3 Analysis and design of single crystal powder synthesis process
3.1 Principle and design of synthesis process
Through the above-mentioned comprehensive study on the control of the particle size, crystal form and nitrogen content of the powder synthesis, a synthesis process is proposed. High-purity C powder and Si powder are selected, and they are evenly mixed and loaded into a graphite crucible according to a silicon-carbon ratio of 1.05. The process steps are mainly divided into four stages:
1) Low-temperature denitrification process, vacuuming to 5×10-4 Pa, then introducing hydrogen, making the chamber pressure about 80 kPa, maintaining for 15 min, and repeating four times. This process can remove nitrogen elements on the surface of carbon powder and silicon powder.
2) High-temperature denitrification process, vacuuming to 5×10-4 Pa, then heating to 950 ℃, and then introducing hydrogen, making the chamber pressure about 80 kPa, maintaining for 15 min, and repeating four times. This process can remove nitrogen elements on the surface of carbon powder and silicon powder, and drive nitrogen in the heat field.
3) Synthesis of low temperature phase process, evacuate to 5×10-4 Pa, then heat to 1350℃, keep for 12 hours, then introduce hydrogen to make the chamber pressure about 80 kPa, keep for 1 hour. This process can remove the nitrogen volatilized during the synthesis process.
4) Synthesis of high temperature phase process, fill with a certain gas volume flow ratio of high purity hydrogen and argon mixed gas, make the chamber pressure about 80 kPa, raise the temperature to 2100℃, keep for 10 hours. This process completes the transformation of silicon carbide powder from β-SiC to α-SiC and completes the growth of crystal particles.
Finally, wait for the chamber temperature to cool to room temperature, fill to atmospheric pressure, and take out the powder.
3.2 Powder post-processing process
After the powder is synthesized by the above process, it must be post-processed to remove free carbon, silicon and other metal impurities and screen the particle size. First, the synthesized powder is placed in a ball mill for crushing, and the crushed silicon carbide powder is placed in a muffle furnace and heated to 450°C by oxygen. The free carbon in the powder is oxidized by heat to generate carbon dioxide gas that escapes from the chamber, thus achieving the removal of free carbon. Subsequently, an acidic cleaning liquid is prepared and placed in a silicon carbide particle cleaning machine for cleaning to remove carbon, silicon and residual metal impurities generated during the synthesis process. After that, the residual acid is washed in pure water and dried. The dried powder is screened in a vibrating screen for particle size selection for crystal growth.
Post time: Aug-08-2024