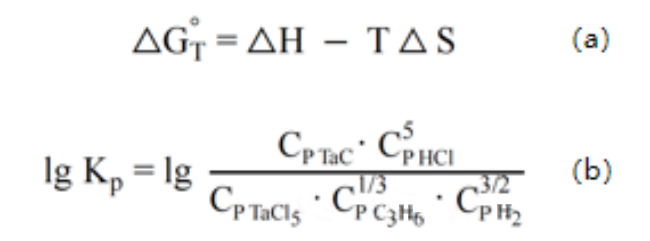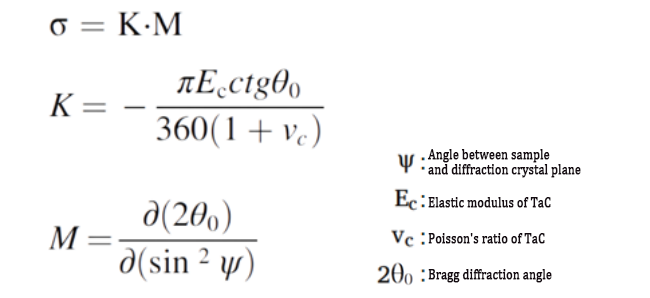I. Process parameter exploration
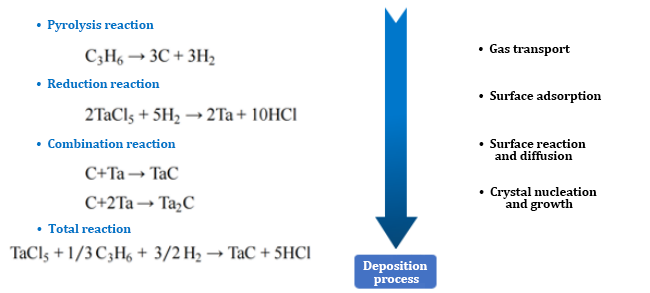
1. TaCl5-C3H6-H2-Ar system
2. Deposition temperature:
According to the thermodynamic formula, it is calculated that when the temperature is greater than 1273K, the Gibbs free energy of the reaction is very low and the reaction is relatively complete. The reaction constant KP is very large at 1273K and increases rapidly with temperature, and the growth rate gradually slows down at 1773K.
Influence on the surface morphology of the coating: When the temperature is not suitable (too high or too low), the surface presents a free carbon morphology or loose pores.
(1) At high temperatures, the movement speed of the active reactant atoms or groups is too fast, which will lead to uneven distribution during the accumulation of materials, and the rich and poor areas cannot smoothly transition, resulting in pores.
(2) There is a difference between the pyrolysis reaction rate of alkanes and the reduction reaction rate of tantalum pentachloride. The pyrolysis carbon is excessive and cannot be combined with tantalum in time, resulting in the surface being wrapped by carbon.
When the temperature is appropriate, the surface of the TaC coating is dense.
TaC particles melt and aggregate with each other, the crystal form is complete, and the grain boundary transitions smoothly.
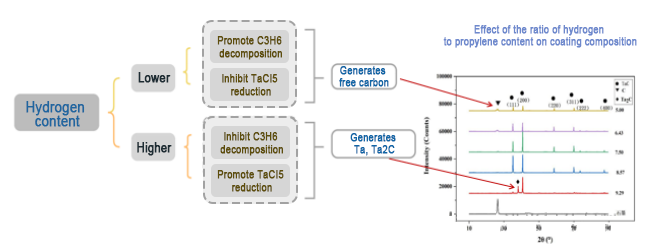
3. Hydrogen ratio:
In addition, there are many factors that affect the coating quality:
-Substrate surface quality
-Deposition gas field
-The degree of uniformity of reactant gas mixing
II. Typical defects of tantalum carbide coating
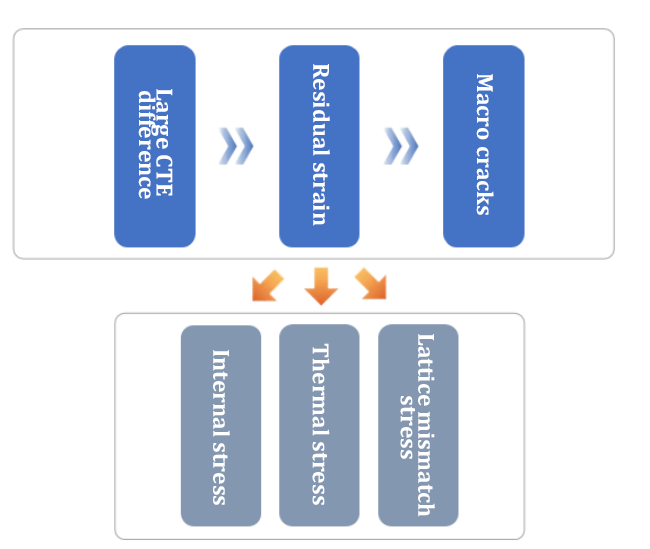
1. Coating cracking and peeling
Linear thermal expansion coefficient linear CTE:
2. Defect analysis:
(1) Cause:
(2) Characterization method
① Use X-ray diffraction technology to measure the residual strain.
② Use Hu Ke’s law to approximate the residual stress.
(3) Related formulas
3.Enhance the mechanical compatibility of the coating and the substrate
(1) Surface in-situ growth coating
Thermal reaction deposition and diffusion technology TRD
Molten salt process
Simplify the production process
Lower the reaction temperature
Relatively lower cost
More environmentally friendly
Suitable for large-scale industrial production
(2) Composite transition coating
Co-deposition process
CVD process
Multi-component coating
Combining the advantages of each component
Flexibly adjust the coating composition and proportion
4. Thermal reaction deposition and diffusion technology TRD
(1) Reaction Mechanism
TRD technology is also called embedding process, which uses boric acid-tantalum pentoxide-sodium fluoride-boron oxide-boron carbide system to prepare tantalum carbide coating.
① Molten boric acid dissolves tantalum pentoxide;
② Tantalum pentoxide is reduced to active tantalum atoms and diffuses on the graphite surface;
③ Active tantalum atoms are adsorbed on the graphite surface and react with carbon atoms to form tantalum carbide coating.
(2) Reaction Key
The type of carbide coating must satisfy the requirement that the oxidation formation free energy of the element forming the carbide is higher than that of boron oxide.
The Gibbs free energy of the carbide is low enough (otherwise, boron or boride may be formed).
Tantalum pentoxide is a neutral oxide. In high-temperature molten borax, it can react with the strong alkaline oxide sodium oxide to form sodium tantalate, thereby reducing the initial reaction temperature.
Post time: Nov-21-2024