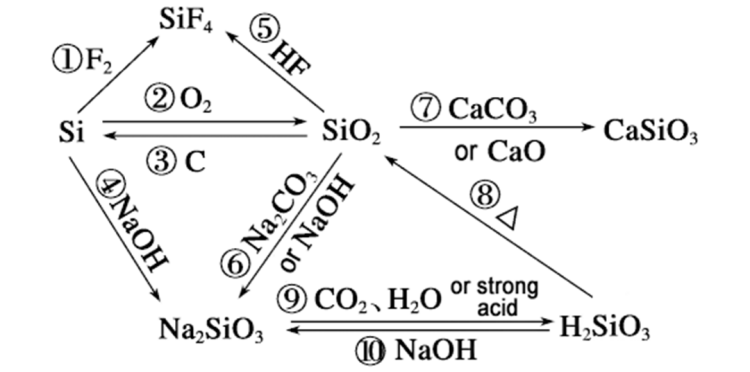
Kungani isilinganiso sokusabela sei-siliconfuthi i-sodium hydroxide ingadlula eye-silicon dioxide ingahlaziywa kulezi zici ezilandelayo:
Umehluko ku-chemical bond energy
▪ Ukusabela kwe-silicon ne-sodium hydroxide: Lapho i-silicon ihlangana ne-sodium hydroxide, amandla ebhondi ye-Si-Si phakathi kwama-athomu e-silicon angu-176kJ/mol kuphela. Isibopho se-Si-Si siyaphuka ngesikhathi sokusabela, okulula ukuphuka. Ngokombono we-kinetic, ukusabela kulula ukuqhubeka.
▪ Ukusabela kwe-silicon dioxide ne-sodium hydroxide: Amandla we-Si-O phakathi kwama-athomu e-silicon nama-athomu omoya-mpilo ku-silicon dioxide angu-460kJ/mol, aphakeme uma kuqhathaniswa. Kuthatha amandla aphezulu ukuphula isibopho se-Si-O ngesikhathi sokusabela, ngakho-ke ukusabela kunzima ukwenzeka futhi izinga lokuphendula lihamba kancane.
Izindlela zokusabela ezihlukene
▪ I-silicon ihlangana ne-sodium hydroxide: I-silicon ihlangana ne-sodium hydroxide kuqala ngokusabela emanzini ukuze ikhiqize i-hydrogen ne-silicic acid, bese i-silicic acid ihlangana ne-sodium hydroxide ukuze ikhiqize i-sodium silicate namanzi. Phakathi nalokhu kusabela, ukusabela phakathi kwe-silicon namanzi kukhipha ukushisa, okungakhuthaza ukunyakaza kwamangqamuzana, ngaleyo ndlela kudale indawo engcono ye-kinetic yokusabela futhi kusheshise izinga lokusabela.
▪ I-Silicon dioxide isabela ne-sodium hydroxide: I-Silicon dioxide ihlangana ne-sodium hydroxide kuqala ngokusabela emanzini ukuze ikhiqize i-silicic acid, bese i-silicic acid ihlangana ne-sodium hydroxide ukuze ikhiqize i-sodium silicate. Ukusabela phakathi kwe-silicon dioxide namanzi kuhamba kancane kakhulu, futhi inqubo yokusabela empeleni ayikukhiphi ukushisa. Ngokombono we-kinetic, ayihambisani nokusabela okusheshayo.
Izakhiwo zezinto ezihlukene
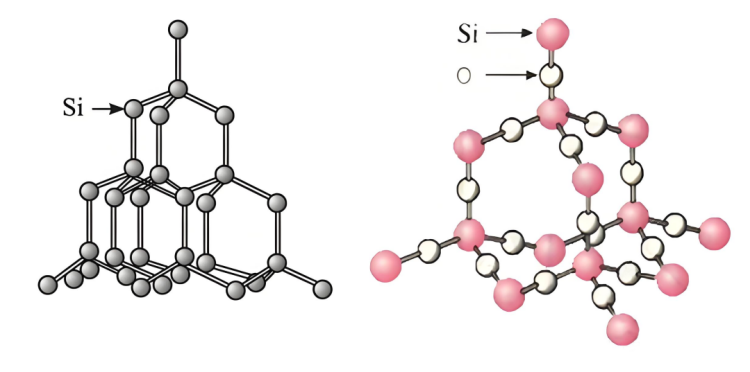
▪ Isakhiwo se-silicon:I-siliconinesakhiwo esithile sekristalu, futhi kukhona izikhala ezithile nokusebenzisana okubuthakathaka kuqhathaniswa phakathi kwama-athomu, okwenza kube lula ngesisombululo se-sodium hydroxide ukuthintana futhi sisabele ngama-athomu e-silicon.
▪ Ukwakheka kwei-siliconi-dioxide:i-siliconI-dioksidi inokwakheka kwenethiwekhi yendawo ezinzile.I-siliconama-athomu nama-athomu omoya-mpilo aboshwe ngokuqinile ngamabhondi ahlangene ukuze akhe isakhiwo sekristalu esiqinile nesiqinile. Kunzima ukuthi isisombululo se-sodium hydroxide singene ngaphakathi kwaso futhi sithinte ngokugcwele ama-athomu e-silicon, okuholela ebunzimeni bokusabela ngokushesha. Ama-athomu e-silicon kuphela angaphezulu kwezinhlayiya ze-silicon dioxide angasabela nge-sodium hydroxide, anciphise izinga lokusabela.
Umthelela wemibandela
▪ Ukusabela kwe-silicon ene-sodium hydroxide: Ngaphansi kwezimo zokushisa, izinga lokusabela kwe-silicon ene-sodium hydroxide solution lizosheshiswa kakhulu, futhi ukusabela ngokuvamile kungaqhubeka kahle emazingeni okushisa aphezulu.
▪ Ukusabela kwe-silicon dioxide ne-sodium hydroxide: Ukusabela kwe-silicon dioxide ene-sodium hydroxide solution kuhamba kancane uma kuqhathaniswa nezinga lokushisa elivamile. Ngokuvamile, izinga lokusabela lizothuthukiswa ngaphansi kwezimo ezinzima ezifana nezinga lokushisa eliphezulu nesisombululo se-sodium hydroxide esigxilile.
Isikhathi sokuthumela: Dec-10-2024