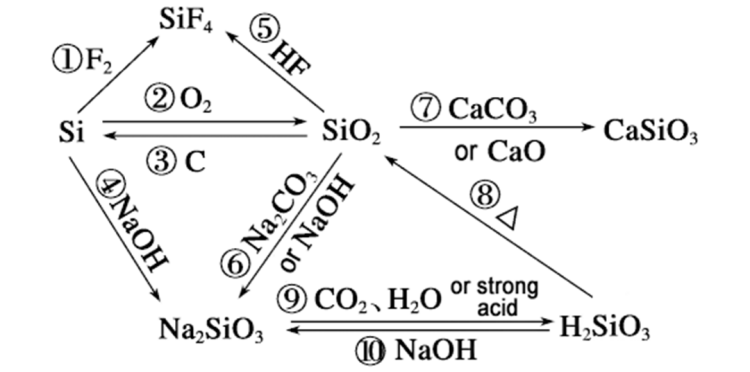
Waa maxay sababta heerka falcelinta eesilikoniyo sodium hydroxide waxay dhaafi kartaa tan silicon dioxide waxaa lagu falanqeyn karaa dhinacyada soo socda:
Farqiga u dhexeeya tamarta curaarta kiimikada
▪ Falcelinta silikoon iyo sodium hydroxide: Marka silikoon ay la falgasho sodium hydroxide, tamarta isku xidhka Si-Si ee u dhaxaysa atamka silikoon waa 176kJ/mol. Dammaanadda Si-Si way jabtaa inta lagu jiro falcelinta, taas oo fudud in la jebiyo. Marka laga eego dhinaca dhaqdhaqaaqa, falcelinta way fududahay in la sii wado.
Falcelinta Silicon dioxide iyo sodium hydroxide: Tamarta isku xidhka Si-O ee u dhaxaysa atamka silikoon iyo atamka ogsijiinta ee silikoon dioxide waa 460kJ/mol, taas oo aad u saraysa. Waxay qaadataa tamar sare si loo jebiyo curaarta Si-O inta lagu jiro falcelinta, markaa falcelintu aad bay u adagtahay inay dhacdo heerka falcelintuna waa gaabis.
Hababka falcelinta kala duwan
Silikoonku waxa uu la falgalaa sodium hydroxide: Silikoon waxa uu la falgalaa sodium hydroxide marka hore isaga oo ku falcelinaya biyo si uu u dhaliyo hydrogen iyo silicic acid, ka dibna silicic acid waxa ay la falgashaa sodium hydroxide si ay u dhaliso sodium silicate iyo biyo. Inta lagu jiro falcelintan, falcelinta u dhaxaysa silikoon iyo biyuhu waxay sii daayaan kulayl, taas oo kor u qaadi karta dhaqdhaqaaqa molecular, taas oo abuuraysa jawi ka wanaagsan falcelinta iyo dardargelinta heerka falcelinta.
Silikon dioxide waxa uu la falgalaa sodium hydroxide: Silikon dioxide waxa uu marka hore la falgalaa sodium hydroxide isaga oo ku falcelinaya biyo si uu u dhaliyo silicic acid, ka dibna silicic acid waxa ay la falgashaa sodium hydroxide si ay u dhaliso sodium silicate. Dareen-celinta u dhaxaysa silikoon ogsaydhsaydh iyo biyuhu aad bay u gaabis tahay, habka fal-celinta asal ahaan ma sii daayo kulayl. Marka laga eego dhinaca dhaqdhaqaaqa, ma aha mid ku habboon falcelinta degdegga ah.
Qaab dhismeedka walxaha kala duwan
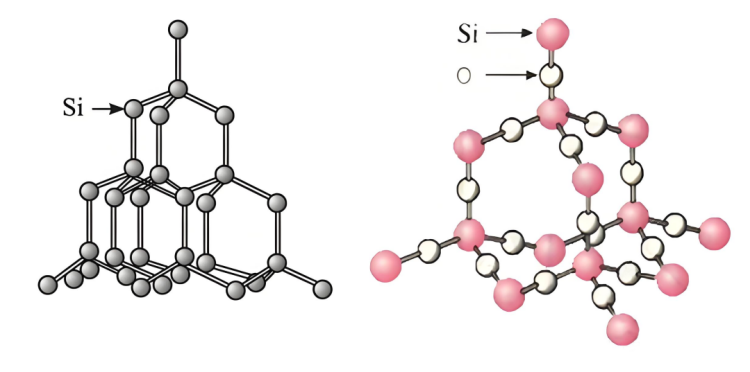
▪ Qaab dhismeedka silikoon:SilikoonWaxay leedahay qaab dhismeed gaar ah, waxaana jira nusqaamo gaar ah iyo isdhexgalka xoogaa daciif ah oo u dhexeeya atamka, taasoo u sahlaysa in xal sodium hydroxide uu la xiriiro oo uu ka falceliyo atamka silikoon.
▪ Qaab dhismeedkasilikondioxide:silikondioxide waxay leedahay qaab-dhismeedka shabakad meel deggan oo deggan.Silikoonatamka iyo atamka ogsijiinta waxaa si adag ugu xidhan bonds covalent si ay u sameeyaan qaab-dhismeedka crystal adag oo xasilloon. Way adag tahay in xal sodium hydroxide uu galo gudaha gudaha oo uu si buuxda ula xiriiro atamka silikoon, taasoo keentay inay ku adkaato falcelinta degdega ah. Kaliya atomyada silikoon ee dusha sare ee qaybaha Silicon dioxide ayaa ka falcelin kara sodium hydroxide, xaddidaya heerka falcelinta.
Saamaynta xaaladaha
▪ Falcelinta silikoon ee leh sodium hydroxide: Xaaladaha kululaynta, heerka falcelinta silikoon ee leh xalka sodium hydroxide si weyn ayaa loo dardar gelin doonaa, falcelintuna guud ahaan waxay ku socon kartaa si habsami leh marka heerkul sare yahay.
▪ Falcelinta Silicon dioxide ee leh sodium hydroxide: Falcelinta silikoon ogsaydh-ku-salaysan ee sodium hydroxide waa mid aad u gaabis ah heerkulka qolka. Caadiyan, heerka falcelinta ayaa lagu wanaajin doonaa marka ay jiraan xaalado adag sida heerkul sare iyo xalalka sodium hydroxide oo la ururiyey.
Waqtiga boostada: Dec-10-2024