Kev nce qib thiab kev txheeb xyuas nyiaj txiag ntawm hydrogen ntau lawm los ntawm electrolysis ntawm cov khoom oxides
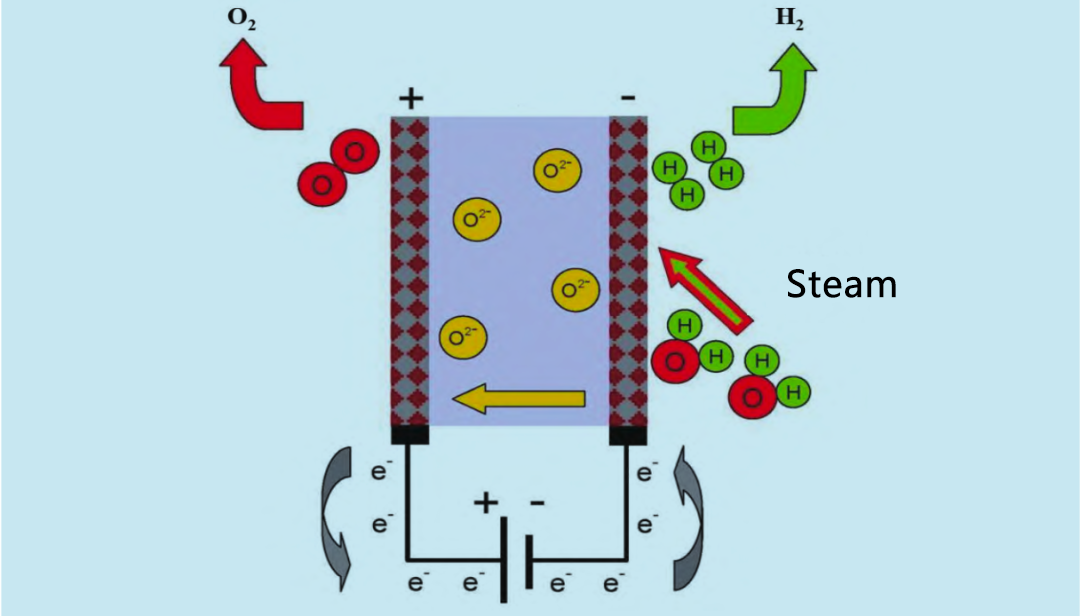
Cov khoom oxide electrolyzer (SOE) siv cov dej kub kub (600 ~ 900 ° C) rau electrolysis, uas yog qhov zoo dua li alkaline electrolyzer thiab PEM electrolyzer. Nyob rau xyoo 1960, Tebchaws Meskas thiab Lub Tebchaws Yelemees tau pib ua kev tshawb fawb txog kev kub siab dej vapor SOE. Lub hauv paus ntsiab lus ua haujlwm ntawm SOE electrolyzer yog pom nyob rau hauv daim duab 4. Recycled hydrogen thiab dej vapor nkag mus rau cov tshuaj tiv thaiv los ntawm anode. Cov dej vapor yog electrolyzed rau hauv hydrogen ntawm lub cathode. Lub O2 tsim los ntawm cathode txav los ntawm cov khoom electrolyte mus rau lub anode, qhov twg nws recombines rau oxygen thiab tso electrons.
Tsis zoo li alkaline thiab proton txauv membrane electrolytic hlwb, SOE electrode reacts nrog dej vapor tiv tauj thiab ntsib qhov kev sib tw ntawm maximizing thaj tsam ntawm lub electrode thiab dej vapor hu. Yog li ntawd, SOE electrode feem ntau muaj cov qauv ntxeem tau. Lub hom phiaj ntawm dej vapor electrolysis yog kom txo tau lub zog siv thiab txo cov nqi khiav hauj lwm ntawm cov pa ua kua dej electrolysis. Qhov tseeb, txawm hais tias tag nrho lub zog xav tau ntawm cov dej decomposition cov tshuaj tiv thaiv nce me ntsis nrog qhov kub thiab txias, qhov xav tau hluav taws xob hluav taws xob txo qis. Raws li qhov kub ntawm electrolytic nce, ib feem ntawm lub zog yuav tsum tau muab los ua cua sov. Lub SOE muaj peev xwm tsim tau hydrogen nyob rau hauv lub xub ntiag ntawm ib tug high-kub kub qhov chaw. Txij li thaum kub-kub roj-txias nuclear reactors tuaj yeem ua kom sov txog 950 ° C, lub zog nuclear tuaj yeem siv los ua lub zog rau SOE. Tib lub sijhawm, kev tshawb fawb qhia tau hais tias lub zog tauj dua tshiab xws li geothermal zog kuj muaj peev xwm ua tau raws li cov cua kub ntawm chav electrolysis. Kev ua haujlwm ntawm qhov kub thiab txias tuaj yeem txo cov roj teeb hluav taws xob thiab nce cov tshuaj tiv thaiv, tab sis nws kuj ntsib kev sib tw ntawm cov khoom siv thermal stability thiab sealing. Tsis tas li ntawd, cov roj uas tsim los ntawm cathode yog cov sib xyaw hydrogen, uas yuav tsum tau muab cais tawm ntxiv thiab ua kom huv, ua kom tus nqi ntau dua piv nrog cov kua dej electrolysis. Kev siv cov proton-conducting ceramics, xws li strontium zirconate, txo tus nqi ntawm SOE. Strontium zirconate qhia tau hais tias zoo heev proton conductivity ntawm txog 700 ° C, thiab yog conducive rau lub cathode los tsim high purity hydrogen, simplifying lub chav electrolysis ntaus ntawv.
Yan et al. [6] tau tshaj tawm tias zirconia ceramic raj ruaj khov los ntawm calcium oxide tau siv los ua SOE ntawm kev txhawb nqa qauv, sab nrauv yog coated nrog nyias (tsawg dua 0.25 hli) ntxeem tau lanthanum perovskite li anode, thiab Ni / Y2O3 ruaj khov calcium oxide cermet li cathode. Ntawm 1000 ° C, 0.4A / cm2 thiab 39.3W input zog, lub peev xwm ntawm hydrogen ntawm chav tsev yog 17.6NL / h. Qhov tsis zoo ntawm SOE yog qhov overvoltage uas tshwm sim los ntawm kev poob siab ohm uas tshwm sim ntawm kev sib tshuam ntawm cov hlwb, thiab qhov siab tshaj qhov siab tshaj vim qhov kev txwv ntawm vapor diffusion thauj. Nyob rau hauv xyoo tas los no, planar electrolytic hlwb tau nyiam heev [7-8]. Nyob rau hauv sib piv rau tubular hlwb, lub hlwb tiaj ua rau kev tsim ntau compact thiab txhim kho hydrogen ntau lawm efficiency [6]. Tam sim no, qhov teeb meem tseem ceeb rau kev lag luam daim ntawv thov ntawm SOE yog lub sij hawm ntev stability ntawm electrolytic cell [8], thiab cov teeb meem ntawm electrode aging thiab deactivation yuav tshwm sim.
Post lub sij hawm: Feb-06-2023