ʻO ka holomua a me ka loiloi waiwai o ka hana hydrogen e ka electrolysis o nā oxide paʻa
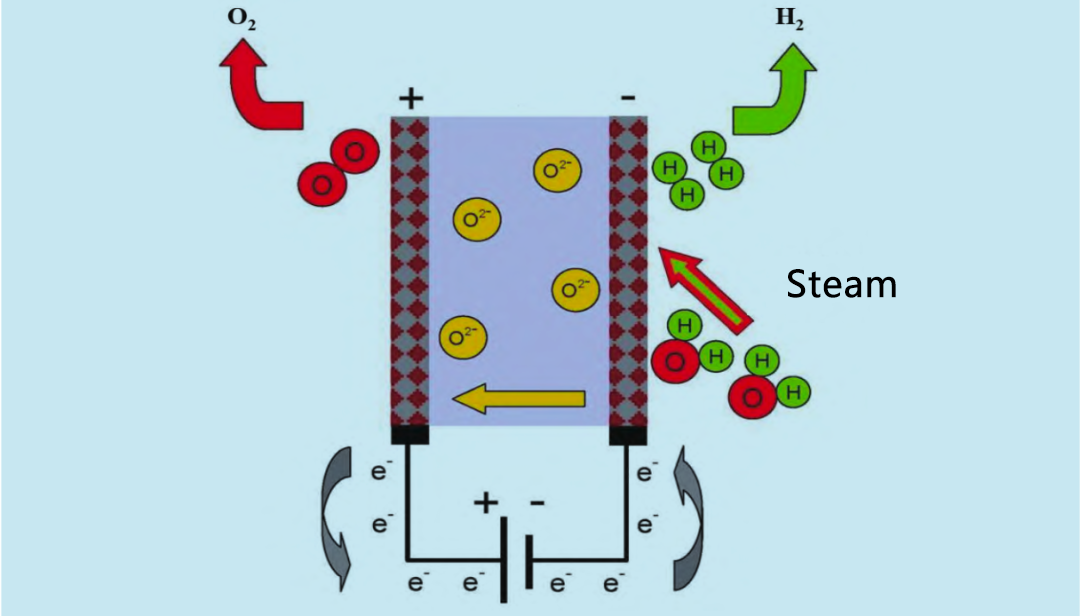
Hoʻohana ka Solid oxide electrolyzer (SOE) i ka mahu wai wela kiʻekiʻe (600 ~ 900 ° C) no ka electrolysis, ʻoi aku ka maikaʻi ma mua o ka electrolyzer alkaline a me ka electrolyzer PEM. I ka makahiki 1960, ua hoʻomaka ʻo ʻAmelika Hui Pū ʻIa a me Kelemānia e hana i ka noiʻi ʻana i ka mahu wai wela SOE. Hōʻike ʻia ke kumu hana o SOE electrolyzer ma ke Kiʻi 4. Hoʻokomo ʻia ka hydrogen a me ka mahu wai i ka ʻōnaehana pane mai ka anode. Hoʻopili ʻia ka mahu wai i ka hydrogen ma ka cathode. ʻO ka O2 i hana ʻia e ka cathode e neʻe ma o ka electrolyte paʻa i ka anode, kahi e hui hou ai e hana i ka oxygen a hoʻokuʻu i nā electrons.
ʻAʻole like me ka alkaline a me ka proton exchange membrane electrolytic cell, ka SOE electrode reacts with water vapor contact and face the challenge of maximizing the interface area between the electrode and water vapor contact. No laila, ʻo ka electrode SOE maʻamau he ʻano porous. ʻO ke kumu o ka electrolysis wai e hoʻemi i ka ikaika o ka ikehu a hōʻemi i ke kumukūʻai hana o ka electrolysis wai wai maʻamau. ʻO ka ʻoiaʻiʻo, ʻoiai ke piʻi iki ka nui o ka ikehu o ka hopena decomposition wai me ka piʻi ʻana o ka mahana, ua emi nui ka pono o ka ikehu uila. Ke piʻi aʻe ka mahana electrolytic, hāʻawi ʻia kahi hapa o ka ikehu e like me ka wela. Hiki i ka SOE ke hana i ka hydrogen i mua o kahi kumu wela wela. No ka mea hiki ke hoʻomehana ʻia nā reactor nuklea hoʻoluʻu kiʻekiʻe i ka 950 ° C, hiki ke hoʻohana ʻia ka ikehu nuklea ma ke ʻano he kumu ikehu no ka SOE. I ka manawa like, hōʻike ka noiʻi ʻana i ka ikehu hou e like me ka ikehu geothermal ka mea hiki ke lilo i kumu wela o ka electrolysis mahu. ʻO ka hana ʻana i ka wela kiʻekiʻe hiki ke hōʻemi i ka volta pālolo a hoʻonui i ka wikiwiki o ka hopena, akā ke alo pū nei i ka paʻakikī o ke kūpaʻa wela a me ka sila. Eia kekahi, ʻo ke kinoea i hana ʻia e ka cathode he hui hydrogen, pono e hoʻokaʻawale hou ʻia a hoʻomaʻemaʻe ʻia, e hoʻonui i ke kumukūʻai i hoʻohālikelike ʻia me ka electrolysis wai wai maʻamau. ʻO ka hoʻohana ʻana i nā seramika proton-conducting, e like me ka strontium zirconate, e hōʻemi i ke kumukūʻai o SOE. Hōʻike ʻo Strontium zirconate i ka conductivity proton maikaʻi loa ma kahi o 700 ° C, a kūpono i ka cathode e hana i ka hydrogen maʻemaʻe kiʻekiʻe, e hoʻomaʻamaʻa i ka mīkini electrolysis mahu.
ʻO Yan et al. [6] hōʻike i ka zirconia ceramic tube stabilized e ka calcium oxide ua hoʻohana ʻia e like me SOE o ke kākoʻo ʻana, ua uhi ʻia ka ʻili o waho me ka lahilahi (emi ma mua o 0.25mm) porous lanthanum perovskite e like me ka anode, a me Ni/Y2O3 stable calcium oxide cermet e like me ka cathode. Ma 1000°C, 0.4A/cm2 a me 39.3W mana hookomo, he 17.6NL/h ka mana hana hydrogen o ka hui. ʻO ka pōʻino o SOE ka overvoltage i loaʻa mai nā poho ohm kiʻekiʻe i maʻamau i nā pilina ma waena o nā pūnaewele, a me ke kiʻekiʻe o ka overvoltage ma muli o nā palena o ka lawe ʻana i ka mahu. I nā makahiki i hala iho nei, ua ʻike nui ʻia nā cell electrolytic planar [7-8]. ʻOkoʻa me nā pūnaewele tubular, ʻoi aku ka paʻakikī o ka hana ʻana a hoʻomaikaʻi i ka hana hydrogen [6]. I kēia manawa, ʻo ka pilikia nui o ka hoʻohana ʻana i ka ʻenehana o SOE ʻo ia ka paʻa lōʻihi o ka cell electrolytic [8], a hiki ke kumu i nā pilikia o ka ʻelemakule electrode a me ka deactivation.
Ka manawa hoʻouna: Feb-06-2023