Ci gaba da nazarin tattalin arziki na samar da hydrogen ta hanyar electrolysis na m oxides
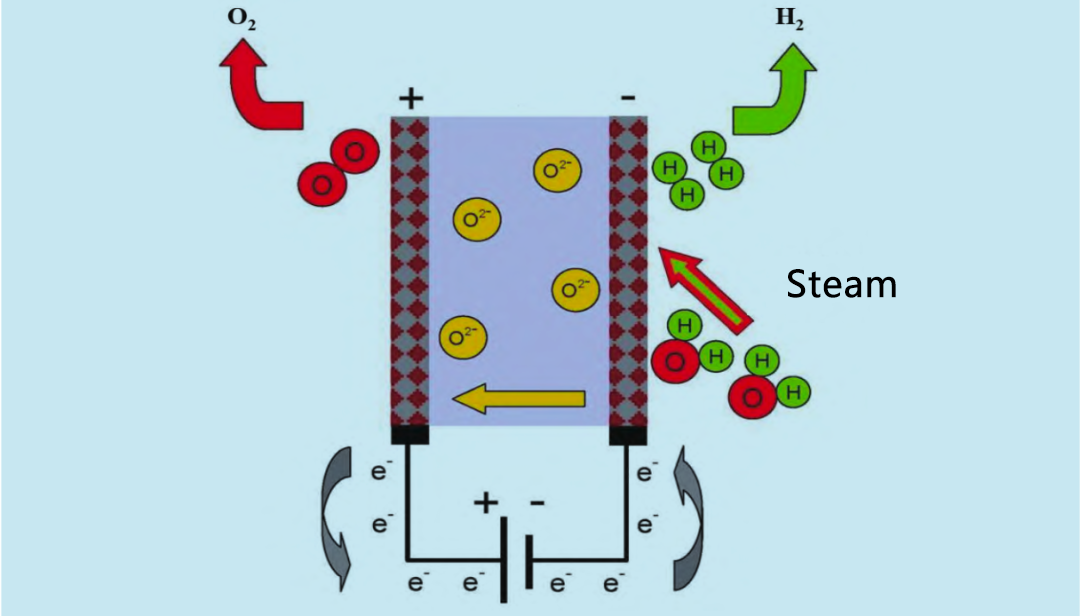
Solid oxide electrolyzer (SOE) yana amfani da tururin ruwa mai zafi (600 ~ 900 ° C) don lantarki, wanda ya fi dacewa fiye da alkaline electrolyzer da PEM electrolyzer. A cikin 1960s, Amurka da Jamus sun fara gudanar da bincike akan tururin ruwa mai zafi SOE. An nuna ka'idar aiki na SOE electrolyzer a cikin Hoto 4. Hydrogen da aka sake yin fa'ida da tururin ruwa sun shiga tsarin amsawa daga anode. Ruwan tururin ruwa yana shiga cikin hydrogen a cathode. O2 da cathode ya samar yana motsawa ta cikin m electrolyte zuwa anode, inda ya sake haɗuwa don samar da oxygen kuma ya saki electrons.
Ba kamar alkaline da proton musayar membrane electrolytic sel ba, SOE electrode yana amsawa tare da tuntuɓar tururi na ruwa kuma yana fuskantar ƙalubalen haɓaka yankin mu'amala tsakanin wutar lantarki da hulɗar tururin ruwa. Saboda haka, SOE lantarki gabaɗaya yana da tsari mara ƙarfi. Manufar ruwa tururin electrolysis shine don rage ƙarfin makamashi da rage farashin aiki na ruwa na ruwa na al'ada. A haƙiƙa, ko da yake jimillar makamashin da ake buƙata na halayen rugujewar ruwa yana ƙaruwa kaɗan tare da ƙara yawan zafin jiki, buƙatun makamashin lantarki yana raguwa sosai. Yayin da zafin jiki na electrolytic ke ƙaruwa, ana ba da ɓangaren makamashin da ake buƙata azaman zafi. SOE na iya samar da hydrogen a gaban tushen zafi mai zafi. Tun da za a iya mai da wutar lantarki mai sanyaya gas mai zafi zuwa 950 ° C, ana iya amfani da makamashin nukiliya azaman tushen makamashi don SOE. A lokaci guda kuma, binciken ya nuna cewa makamashin da ake iya sabuntawa kamar makamashin geothermal shima yana da yuwuwar tushen zafi na wutar lantarki. Yin aiki a babban zafin jiki na iya rage ƙarfin baturi da haɓaka ƙimar amsawa, amma kuma yana fuskantar ƙalubalen kwanciyar hankali na kayan zafi da rufewa. Bugu da ƙari, iskar gas ɗin da cathode ke samarwa shine cakuda hydrogen, wanda ke buƙatar ƙarin rabuwa da tsarkakewa, yana ƙaruwa da farashi idan aka kwatanta da na al'ada na ruwa na ruwa. Yin amfani da yumbu mai sarrafa proton, irin su strontium zirconate, yana rage farashin SOE. Strontium zirconate yana nuna kyakkyawan halayen proton a kusan 700 ° C, kuma yana dacewa da cathode don samar da babban tsaftar hydrogen, yana sauƙaƙe na'urar lantarki ta tururi.
Yan et al. [6] ya ruwaito cewa zirconia yumbu tube stabilized ta calcium oxide da aka yi amfani da SOE na goyon bayan tsarin, da waje surface da aka mai rufi da bakin ciki (kasa da 0.25mm) porous lanthanum perovskite a matsayin anode, da Ni / Y2O3 barga calcium oxide cermet a matsayin cathode. A 1000 ° C, 0.4A/cm2 da 39.3W ikon shigarwa, ƙarfin samar da hydrogen na naúrar shine 17.6NL / h. Rashin lahani na SOE shine yawan wutar lantarki da ke haifar da asarar ohm mai yawa wanda ya zama ruwan dare a haɗin kai tsakanin sel, da kuma yawan yawan ƙarfin lantarki saboda iyakancewar jigilar tururi. A cikin 'yan shekarun nan, sel electrolytic planar sun jawo hankali sosai [7-8]. Ya bambanta da sel tubular, sel masu lebur suna sa masana'anta ƙarami da haɓaka haɓakar samar da hydrogen [6]. A halin yanzu, babban cikas ga aikace-aikacen masana'antu na SOE shine kwanciyar hankali na dogon lokaci na tantanin halitta [8], kuma ana iya haifar da matsalolin tsufa na lantarki da kashewa.
Lokacin aikawa: Fabrairu-06-2023