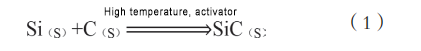A cikin tsarin haɓakar kristal silicon carbide guda ɗaya, jigilar tururi ta zahiri shine hanyar masana'antu ta yau da kullun. Don hanyar haɓakar PVT,siliki carbide fodayana da tasiri mai girma akan tsarin girma. Duk sigogi nasiliki carbide fodakai tsaye yana shafar ingancin ci gaban kristal guda ɗaya da kaddarorin lantarki. A cikin aikace-aikacen masana'antu na yanzu, waɗanda aka saba amfani da susiliki carbide fodaTsarin kira shine hanyar haɗin kai mai zafi mai zafi.
Hanyar haɓaka yanayin zafin jiki mai kai da kai tana amfani da zafin jiki mai ƙarfi don ba masu amsawa zafin farko don fara halayen sinadarai, sannan kuma suyi amfani da nasu zafin halayen sinadarai don ba da damar abubuwan da ba a yi su ba su ci gaba da kammala aikin sinadarai. Duk da haka, tun da sinadarai na Si da C suna sakin zafi kaɗan, dole ne a ƙara wasu masu amsawa don kula da halayen. Don haka, malamai da yawa sun ba da shawarar ingantacciyar hanyar haɗin kai ta wannan tushe, suna gabatar da mai kunnawa. Hanyar yada kai tana da sauƙin aiwatarwa, kuma sigogin haɗawa daban-daban suna da sauƙin sarrafawa. Haɗin kai mai girma yana biyan bukatun masana'antu.
A farkon 1999, Bridgeport yayi amfani da hanyar haɓaka yanayin zafi mai ƙarfi don haɗawa.SiC foda, amma ya yi amfani da ethoxysilane da phenol resin a matsayin albarkatun kasa, wanda ya kasance mai tsada. Gao Pan da sauransu sun yi amfani da Si foda mai tsabta da C foda a matsayin albarkatun kasa don haɗawaSiC fodaby high-zazzabi dauki a cikin wani argon yanayi. Ning Lina ya shirya babban-barbashiSiC fodata hanyar haɗakarwa ta sakandare.
Matsakaicin mitar induction dumama tanderun da Cibiyar Bincike ta Biyu ta China Electronics Technology Group Corporation ta haɓaka a ko'ina tana haɗa foda silicon da foda carbon a cikin wani nau'i na stoichiometric kuma yana sanya su a cikin ma'aunin graphite. Thegraphite crucibleana sanya shi a cikin tanderun dumama shigar da matsakaici-mita don dumama, kuma ana amfani da canjin zafin jiki don haɗawa da canza yanayin ƙarancin zafin jiki da babban yanayin yanayin silicon carbide bi da bi. Tun da yanayin zafin β-SiC na haɗin kai a cikin ƙananan yanayin zafi ya fi ƙasa da yanayin zafin jiki na Si, haɗin β-SiC a ƙarƙashin babban injin zai iya tabbatar da yaduwar kai. Hanyar gabatar da argon, hydrogen da HCl gas a cikin kira na α-SiC yana hana bazuwarSiC fodaa cikin babban yanayin zafi, kuma zai iya rage yawan abun ciki na nitrogen a cikin α-SiC foda.
Shandong Tianyue ya ƙera tanderun haɗaɗɗiya, ta amfani da iskar silane azaman kayan albarkatun siliki da foda carbon azaman albarkatun carbon. Adadin iskar gas da aka gabatar an daidaita shi ta hanyar haɗakar matakai biyu, kuma girman ƙwayar siliki na carbide na ƙarshe ya kasance tsakanin 50 da 5 000 um.
1 Abubuwan sarrafawa na tsarin tsarin foda
1.1 Tasirin girman ƙwayar foda akan ci gaban crystal
Girman barbashi na siliki carbide foda yana da tasiri mai mahimmanci akan ci gaban kristal guda ɗaya na gaba. Ci gaban SiC guda kristal ta hanyar PVT ana samun galibi ta hanyar canza madaidaicin ma'aunin siliki da carbon a cikin ɓangaren gas, kuma rabon molar silicon da carbon a cikin ɓangaren gas ɗin yana da alaƙa da girman barbashi na siliki carbide foda. Jimlar matsa lamba da rabon siliki-carbon na tsarin haɓaka yana ƙaruwa tare da raguwar girman barbashi. Lokacin da girman barbashi ya ragu daga 2-3 mm zuwa 0.06 mm, rabon siliki-carbon yana ƙaruwa daga 1.3 zuwa 4.0. Lokacin da barbashi sun yi ƙanƙanta zuwa wani matsayi, matsa lamba na Si yana ƙaruwa, kuma an kafa wani Layer na fim ɗin a saman kristal mai girma, yana haifar da haɓakar gas-ruwa mai ƙarfi, wanda ke shafar polymorphism, lahani da lahani na layi a cikin crystal. Don haka, dole ne a sarrafa girman ƙwayar silikon carbide foda mai tsafta.
Bugu da ƙari, lokacin da girman ƙwayar SiC foda yana da ƙananan ƙananan, foda yana raguwa da sauri, yana haifar da girma mai girma na SiC guda lu'ulu'u. A gefe guda, a cikin yanayin zafi mai zafi na SiC guda kristal girma, ana aiwatar da matakai biyu na haɓakawa da lalata lokaci guda. Silicon carbide foda zai bazuwa da samar da carbon a cikin gas lokaci da m lokaci irin su Si, Si2C, SiC2, sakamakon da tsanani carbonization na polycrystalline foda da kuma samuwar carbon inclusions a cikin crystal; a gefe guda, lokacin da adadin raguwa na foda ya kasance da sauri, tsarin crystal na SiC guda kristal mai girma yana da wuyar canzawa, yana da wuya a sarrafa ingancin SiC mai girma.
1.2 Tasirin foda crystal form akan ci gaban crystal
Girman SiC guda crystal ta hanyar PVT shine tsarin sublimation-recrystallization a babban zafin jiki. Siffar kristal na SiC albarkatun kasa yana da tasiri mai mahimmanci akan ci gaban crystal. A cikin aiwatar da haɗin foda, ƙananan zafin jiki na kira (β-SiC) tare da tsarin cubic na tantanin halitta da kuma yanayin yanayin zafi mai zafi (α-SiC) tare da tsarin hexagonal na tantanin halitta za a samar da shi. Akwai nau'ikan siliki carbide crystal da yawa da kewayon sarrafa zafin jiki kunkuntar. Misali, 3C-SiC zai canza zuwa silicon carbide polymorph hexagonal, watau 4H/6H-SiC, a yanayin zafi sama da 1900°C.
A lokacin tsarin ci gaban kristal guda ɗaya, lokacin da ake amfani da β-SiC foda don haɓaka lu'ulu'u, ƙimar siliki-carbon molar rabo ya fi 5.5, yayin da ake amfani da α-SiC foda don girma lu'ulu'u, ƙimar molar silicon-carbon shine 1.2. Lokacin da zafin jiki ya tashi, canjin lokaci yana faruwa a cikin crucible. A wannan lokacin, rabon molar a cikin lokaci na gas ya zama mafi girma, wanda ba shi da amfani ga ci gaban crystal. Bugu da ƙari, sauran ƙazantar iskar gas, da suka haɗa da carbon, silicon, da silicon dioxide, ana samun su cikin sauƙi yayin aiwatar da canjin lokaci. Kasancewar waɗannan ƙazanta yana haifar da kristal don haifar da microtubes da voids. Saboda haka, foda crystal form dole ne a sarrafa daidai.
1.3 Tasirin ƙazantattun foda akan ci gaban crystal
Abubuwan da ke cikin ƙazanta a cikin SiC foda yana rinjayar ƙwayar cuta ta hanzari yayin girma crystal. Mafi girman abun ciki na ƙazanta, ƙarancin yuwuwar shine don crystal ɗin ya yi kwatsam. Don SiC, manyan ƙazantattun ƙarfe sun haɗa da B, Al, V, da Ni, waɗanda za a iya gabatar da su ta hanyar kayan aikin sarrafawa yayin sarrafa foda na silicon da foda na carbon. Daga cikin su, B da Al sune manyan ƙazantattun masu karɓar matakin makamashi mara ƙarancin ƙarfi a cikin SiC, wanda ke haifar da raguwar tsayayyar SiC. Sauran ƙazanta na ƙarfe za su gabatar da matakan makamashi da yawa, wanda ke haifar da kaddarorin lantarki marasa ƙarfi na SiC guda lu'ulu'u a yanayin zafi mai yawa, kuma suna da tasiri mafi girma akan kaddarorin lantarki na tsattsauran tsafta na tsaka-tsakin tsaka-tsakin kristal guda ɗaya, musamman tsayayya. Sabili da haka, dole ne a haɗa foda mai tsabta na silicon carbide kamar yadda zai yiwu.
1.4 Tasirin abun ciki na nitrogen a cikin foda akan ci gaban crystal
Matsayin abun ciki na nitrogen yana ƙayyadaddun juriya na ƙwayar kristal guda ɗaya. Manyan masana'antun suna buƙatar daidaita ma'aunin doping na nitrogen a cikin kayan roba bisa ga tsarin haɓakar kristal balagagge yayin haɗin foda. Babban tsaftataccen siliki-carbide guda kristal substrates sune mafi kyawun kayan don kayan aikin kayan lantarki na soja. Don girma babban-tsarki na tsaka-tsakin tsaka-tsakin kristal guda ɗaya tare da babban juriya da kyawawan kaddarorin lantarki, abun ciki na babban ƙazanta nitrogen a cikin ƙasa dole ne a sarrafa shi a ƙaramin matakin. Abubuwan da ake amfani da su na kristal guda ɗaya suna buƙatar abun ciki na nitrogen don sarrafa su a wani babban taro.
2 Fasaha mai sarrafa maɓalli don haɗin foda
Saboda daban-daban yanayin amfani da silicon carbide substrates, da kira fasahar for girma powders kuma yana da daban-daban matakai. Don nau'in nau'in nau'in nau'in ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar ƙwayar cuta, ana buƙatar babban tsabta da tsabta da kuma lokaci guda; yayin da Semi-insulating guda kristal girma foda, ana buƙatar tsananin kulawa da abun ciki na nitrogen.
2.1 Foda size iko
2.1.1 zafin jiki na haɗin gwiwa
Tsayawa sauran yanayin tsari ba canzawa, SiC powders generated a kira yanayin zafi na 1900 ℃, 2000 ℃, 2100 ℃, da 2200 ℃ aka sampled da kuma nazari. Kamar yadda aka nuna a cikin Figure 1, ana iya ganin cewa girman barbashi shine 250 ~ 600 μm a 1900 ℃, kuma girman barbashi yana ƙaruwa zuwa 600 ~ 850 μm a 2000 ℃, kuma girman ƙwayar ya canza sosai. Lokacin da yawan zafin jiki ya ci gaba da tashi zuwa 2100 ℃, girman barbashi na SiC foda shine 850 ~ 2360 μm, kuma karuwa yana jin dadi. Girman barbashi na SiC a 2200 ℃ yana da ƙarfi a kusan 2360 μm. Ƙara yawan zafin jiki na kira daga 1900 ℃ yana da tasiri mai kyau akan girman ƙwayar SiC. Lokacin da zafin jiki na kira ya ci gaba da karuwa daga 2100 ℃, girman barbashi ba ya canzawa sosai. Sabili da haka, lokacin da aka saita yawan zafin jiki na kira zuwa 2100 ℃, za'a iya haɗa girman girman ƙwayar ƙwayar cuta a ƙananan makamashi.
2.1.2 Lokacin hadawa
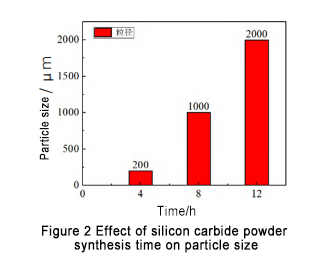
Sauran yanayin tsari ba su canzawa, kuma an saita lokacin kira zuwa 4 h, 8h, da 12 h bi da bi. Ana nuna samfurin samfurin SiC foda da aka samar a cikin Hoto 2. An gano cewa lokacin kira yana da tasiri mai mahimmanci akan girman ƙwayar SiC. Lokacin da lokacin kira shine 4 h, ana rarraba nau'in nau'in nau'in a 200 μm; Lokacin da synthesis lokacin shine 8 h, girman ƙwayar ƙwayar ƙwayar cuta ta ƙara muhimmanci sosai, galibi rarraba a kusan 1 000 μm; yayin da lokacin kira ya ci gaba da ƙaruwa, girman ƙwayar ƙwayar cuta yana ƙaruwa, yawanci ana rarrabawa a kusan 2 000 μm.
2.1.3 Tasirin girman ɓangarorin albarkatun ƙasa
Yayin da sarkar samar da kayan siliki na cikin gida ke haɓaka sannu a hankali, ana ƙara haɓaka tsabtar kayan siliki. A halin yanzu, kayan siliki da ake amfani da su wajen haɗawa an raba su zuwa silikon granular da silikon foda, kamar yadda aka nuna a hoto na 3.
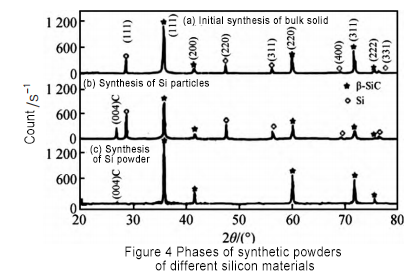
An yi amfani da albarkatun siliki daban-daban don gudanar da gwaje-gwajen haɗakar silicon carbide. Ana nuna kwatancen samfuran roba a cikin Hoto 4. Bincike ya nuna cewa lokacin amfani da toshe albarkatun siliki, babban adadin abubuwan Si yana cikin samfurin. Bayan da aka murkushe toshe silicon a karo na biyu, sinadarin Si a cikin kayan roba yana raguwa sosai, amma har yanzu yana nan. A ƙarshe, ana amfani da foda na silicon don haɓakawa, kuma SiC kawai yana cikin samfurin. Wannan shi ne saboda a cikin samar da tsari, babban-size granular silicon bukatar fara fara da surface kira dauki, da kuma silicon carbide aka hada a kan surface, wanda ya hana ciki Si foda daga kara hadawa da C foda. Sabili da haka, idan ana amfani da toshe silicon azaman albarkatun ƙasa, yana buƙatar murkushe sa'an nan kuma a sanya shi zuwa tsarin haɗakarwa na biyu don samun foda na silicon carbide don haɓakar crystal.
2.2 Foda crystal tsari iko
2.2.1 Tasirin zafin jiki na kira
Tsayawa da sauran yanayin tsari bai canza ba, yawan zafin jiki na kira shine 1500 ℃, 1700 ℃, 1900 ℃, da 2100 ℃, kuma ana yin samfurin SiC foda da aka yi nazari. Kamar yadda aka nuna a cikin Hoto 5, β-SiC rawaya ce ta ƙasa, kuma α-SiC ya fi sauƙi a launi. Ta lura da launi da ilimin halittar jiki na hada foda, za a iya ƙaddara cewa hada samfurin ne β-SiC a yanayin zafi na 1500 ℃ da 1700 ℃. A 1900 ℃, launi ya zama mai sauƙi, kuma ƙwayoyin hexagonal sun bayyana, yana nuna cewa bayan zafin jiki ya tashi zuwa 1900 ℃, wani lokaci na canzawa yana faruwa, kuma wani ɓangare na β-SiC ya canza zuwa α-SiC; lokacin da zafin jiki ya ci gaba da tashi zuwa 2100 ℃, an gano cewa ƙwayoyin da aka haɗa su ne m, kuma α-SiC an canza shi.
2.2.2 Tasirin lokacin kira
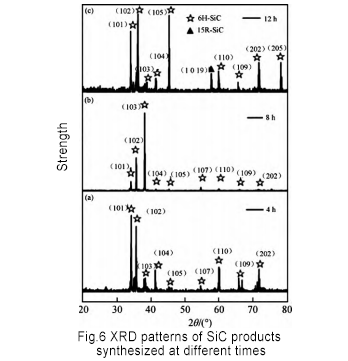
Sauran yanayin tsari ba su canzawa, kuma an saita lokacin kira zuwa 4h, 8h, da 12h, bi da bi. An ƙirƙiri foda na SiC da aka yi amfani da shi kuma an bincika shi ta diffractometer (XRD). Ana nuna sakamakon a cikin Hoto 6. Lokacin kira yana da wani tasiri akan samfurin da SiC foda ya hada. Lokacin da lokacin haɗawa shine 4h da 8h, samfurin roba shine yafi 6H-SiC; lokacin da lokacin kira shine 12h, 15R-SiC yana bayyana a cikin samfurin.
2.2.3 Tasirin rabon albarkatun kasa
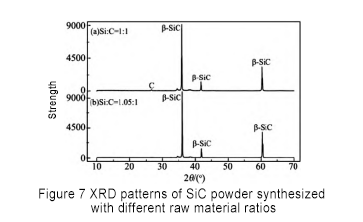
Sauran hanyoyin ba su canzawa, ana nazarin adadin abubuwan siliki-carbon, kuma ma'auni sune 1.00, 1.05, 1.10 da 1.15 bi da bi don gwaje-gwajen kira. Ana nuna sakamakon a hoto na 7.
Daga bakan XRD, ana iya ganin cewa lokacin da rabon silicon-carbon ya fi 1.05, wuce haddi Si ya bayyana a cikin samfurin, kuma lokacin da rabon silicon-carbon bai wuce 1.05 ba, wuce haddi C yana bayyana. Lokacin da rabon siliki-carbon ya kasance 1.05, carbon ɗin kyauta a cikin samfuran roba yana kawar da gaske, kuma babu siliki na kyauta ya bayyana. Saboda haka, adadin rabon siliki-carbon rabo ya kamata ya zama 1.05 don haɗa SiC mai tsafta.
2.3 Sarrafa ƙananan abun ciki na nitrogen a cikin foda
2.3.1 Rubutun albarkatun kasa
Kayan albarkatun da aka yi amfani da su a cikin wannan gwaji sune babban foda na carbon foda da siliki mai tsabta mai tsabta tare da matsakaicin diamita na 20 μm. Saboda ƙananan ƙananan ƙwayoyin su da kuma ƙayyadaddun yanki na musamman, suna da sauƙin sha N2 a cikin iska. Lokacin da ake hada foda, za a kawo shi cikin nau'in crystal na foda. Don haɓakar nau'in lu'ulu'u N, rashin daidaituwa na doping na N2 a cikin foda yana haifar da juriya mara daidaituwa na crystal har ma da canje-canje a cikin sigar crystal. Abubuwan da ke cikin nitrogen na foda da aka haɗa bayan an gabatar da hydrogen yana da ƙasa sosai. Wannan shi ne saboda ƙarar ƙwayoyin hydrogen ƙanana ne. Lokacin da N2 adsorbed a cikin carbon foda da silicon foda ne mai tsanani da bazuwa daga saman, H2 da cikakken yaduwa a cikin rata tsakanin foda tare da karamin girma, maye gurbin da matsayi na N2, da kuma N2 tserewa daga crucible a lokacin vacuum tsari, cimma manufar cire nitrogen abun ciki.
2.3.2 Tsarin aiki
A lokacin da ake kira silicon carbide foda, tun da radius na carbon atoms da nitrogen atoms yayi kama da haka, nitrogen zai maye gurbin guraben carbon a cikin silicon carbide, don haka yana ƙara yawan abun ciki na nitrogen. Wannan tsari na gwaji yana ɗaukar hanyar gabatar da H2, kuma H2 yana amsawa tare da abubuwan carbon da silicon a cikin haɗakarwa don samar da iskar C2H2, C2H, da SiH. Abubuwan da ke cikin sinadarin carbon yana ƙaruwa ta hanyar watsa lokaci na iskar gas, ta haka yana rage guraben carbon. An cimma manufar cire nitrogen.
2.3.3 Tsarin sarrafa abun ciki na nitrogen a bango
Za'a iya amfani da ƙwanƙwasa graphite tare da babban porosity azaman ƙarin tushen C don shayar da Si tururi a cikin sassan lokaci na iskar gas, rage Si a cikin abubuwan haɗin gas, don haka ƙara C / Si. A lokaci guda kuma, graphite crucibles kuma na iya amsawa tare da yanayin Si don samar da Si2C, SiC2 da SiC, wanda yayi daidai da yanayin Si yana kawo tushen C daga graphite crucible zuwa yanayin girma, yana haɓaka rabon C, da haɓaka rabon carbon-silicon. Sabili da haka, ana iya haɓaka rabon carbon-silicon ta hanyar amfani da crucibles graphite tare da babban porosity, rage guraben carbon, da cimma manufar cire nitrogen.
3 Analysis da zane na guda crystal foda kira tsari
3.1 Ƙa'ida da ƙira na tsarin kira
Ta hanyar bincike mai zurfi da aka ambata a sama akan kula da girman nau'in, nau'in crystal da abun ciki na nitrogen na foda kira, an gabatar da tsarin kira. An zaɓi foda mai tsaftar C da Si foda, kuma ana haɗa su daidai gwargwado kuma an ɗora su a cikin faifan graphite bisa ga rabon silicon-carbon na 1.05. Matakan tsari sun kasu galibi zuwa matakai hudu:
1) Tsarin denitrification na ƙananan zafin jiki, vacuuming zuwa 5 × 10-4 Pa, sa'an nan kuma gabatar da hydrogen, yin matsin lamba game da 80 kPa, kiyayewa na 15 min, da maimaita sau hudu. Wannan tsari zai iya cire abubuwan nitrogen a saman carbon foda da siliki foda.
2) Tsarin denitrification mai zafi mai zafi, vacuuming zuwa 5 × 10-4 Pa, sa'an nan kuma dumama zuwa 950 ℃, sa'an nan kuma gabatar da hydrogen, yin matsa lamba na ɗakin game da 80 kPa, kiyayewa na 15 min, da maimaita sau hudu. Wannan tsari zai iya cire abubuwan nitrogen a saman carbon foda da siliki foda, da kuma fitar da nitrogen a cikin filin zafi.
3) Kira na ƙananan zafin jiki lokaci tsari, ƙaura zuwa 5 × 10-4 Pa, sa'an nan zafi zuwa 1350 ℃, ci gaba da 12 hours, sa'an nan gabatar da hydrogen su sa dakin matsa lamba game da 80 kPa, kiyaye for 1 hour. Wannan tsari zai iya cire nitrogen wanda aka canza a lokacin aikin kira.
4) Kira na high zafin jiki lokaci tsari, cika da wani gas girma kwarara rabo daga high tsarki hydrogen da argon gauraye gas, sa dakin matsa lamba game da 80 kPa, tãyar da zazzabi zuwa 2100 ℃, ci gaba da 10 hours. Wannan tsari ya kammala canjin siliki carbide foda daga β-SiC zuwa α-SiC kuma ya kammala haɓakar ƙwayoyin crystal.
A ƙarshe, jira zafin ɗakin ya yi sanyi zuwa zafin ɗaki, cika matsi na yanayi, kuma cire foda.
3.2 Foda bayan aiwatarwa tsari
Bayan an haɗa foda ta hanyar tsarin da ke sama, dole ne a aiwatar da shi don cire carbon, silicon da sauran ƙazantattun ƙarfe na kyauta da allon girman ɓangaren. Da farko, ana sanya foda da aka haɗe a cikin injin niƙa don murƙushewa, kuma ana sanya foda na siliki carbide da aka murƙushe a cikin tanderun murfi da zafi zuwa 450 ° C ta iskar oxygen. Carbon kyauta a cikin foda yana oxidized da zafi don samar da iskar carbon dioxide da ke tserewa daga ɗakin, don haka cimma kawar da carbon kyauta. Daga baya, an shirya ruwa mai tsaftar acidic kuma an sanya shi a cikin injin tsabtace barbashi na siliki don tsaftacewa don cire carbon, silicon da sauran ƙazantattun ƙarfe waɗanda aka haifar yayin aikin haɗin gwiwa. Bayan haka, ana wanke ragowar acid a cikin ruwa mai tsabta kuma a bushe. Ana duba busasshen foda a cikin allo mai girgiza don zaɓin girman barbashi don ci gaban crystal.
Lokacin aikawa: Agusta-08-2024